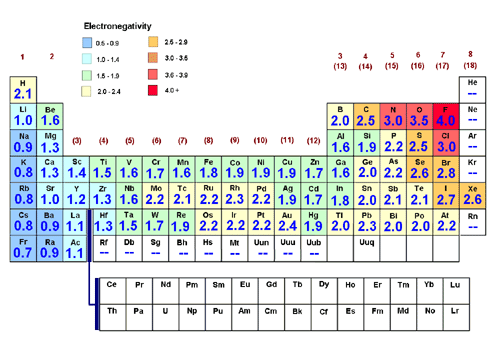
Most elements want to have a complete electron shell with 8 electrons. Chemists have been able to calculate their electronegativities as Ar 32 Kr 30 Xe 26 Rn 26.
Why Do Noble Gases Have No Established Electronegativities Quora
For all noble gases at the time Pauling couldnt have known.

. This is because all of the Noble Gases have complete valence electron shells. This shows they are in group 8 meaning they have 8 electrons in their outermost energy level. Noble gases do have an electronegativity.
Ca Se As Br. But the calculation of electronegativity from experimental data is a bit complicated. The Noble gases like helium neon and argon all have full valence shells.
All the noble gases except helium and neon form compounds with highly electronegative elements. Since electronegativity measures the amount of attraction between an atom and an electron noble gases do not have electronegativity. Think of 8 as being the best car that everyone wants.
They are gases 4. Learn vocabulary terms and more with flashcards games and other study tools. This is the reason why noble gases usually have very large ionization energies.
It is just extremely unlikely. Why do noble gases have no electronegativity. All noble gases have stable outer electron shells.
They have a complete set of 8 electrons. So really its more accurate to say that the noble gases under the Pauling definition of electronegativity mostly have undefined electronegativity values. As seen from the Periodic Table the noble gases are located at the far right.
They dont have values there because they arent on the pauling scale of electronegativity as they dont form any compounds with other elements. Arrange in order of increasing electronegativity. Electronegativity is a measurement of the power of an atom to attract electrons.
However in the 1960s researchers found that ceKr and ceXe did react with extremely electronegative elements such as fluorine and oxygen to form new molecules. Since the noble gases already have eight electrons in their outer shells they dont want to attract any more. Since noble gases have completely filled valence shells that are highly stable a lot of energy would be required to remove even a single electron from the valence shell of a noble gas.
They are generally unreactive noble because all of their occupied orbitals are filled with electrons - they really dont want to gain or lose an electron. Thus he couldnt find values for them. Ionization energy can be thought of as the energy required to remove an electron from the valence shell of an atom.
Pauling just didnt have them at first because one thing he needed was homonuclear bond energies. Since the Noble Gases already have that. The bond enthalpy of an A-B bond.
No value Se S O. All of the Noble Gases actually have electron affinities of less than or equal to 0. Values for the noble gasses He Ne Ar and Rn are listed as NA not applicable.
For example there is a compound called Argon Fluorohydride HArF that can exist but is only. You should probably say that a noble gas has zero electronegativity because they dont form bonds and therefore must not want electrons. However argon and neon can technically form compounds with other elements.
This means they are very stable and have no need to bond. The atoms of noble gases already have complete outer shells so they have no tendency to lose gain or share electrons. These noble gasses wont bond.
Noble gases have completely filled electronic configuration and hence they are chemically inactive inert and have no electronegativity. Why dont noble gases have electronegativity values. This is why the noble gases are inert and do not take part in chemical.
They only form compounds with non-metals 3. The bond enthalpy of an B-B bond. Combined with the ionization energies in good agreement with experiment an electronegativity scale is obtained displaying 1 a monotonic decrease of χ when going down the periodic table 2 top values not for the noble gases but for the halogens as opposed to most extrapolation procedures of existing scales invariably placing the noble gases on top and 3 noble gases.
Correct answer to the question Why dont noble gases have a value for electronegativity. They only form compounds with metals 2. Start studying Electronegativity Periodic Trends.
Answer 1 of 3. Noble gases are not counted for their electronegativity because unlike the other elements they have a perfect octet so it would be unfavorable for them to gain another electron.
Why Are The Electronegativity Values Of Noble Gases Zero Socratic
Why Does Helium Have No Electronegativity Quora

Question Video Understanding Why Some Noble Gas Elements Do Not Have Electronegativity Values Nagwa

Illustration About Periodic Table Of Elements With Electronegativity Values Illustration Of Elements Ele Printable Chart Chemistry Study Guide Periodic Table
0 Comments